Subtotal £0.00
Subscribe to out newsletter today to receive latest news.
2478 Street City Ohio 90255
Shopping cart
- Phone:+44 7490 342983
- Email:info@micjeffonpharmacy.com
- United Kingdom
Subscribe to out newsletter today to receive latest news.
2478 Street City Ohio 90255
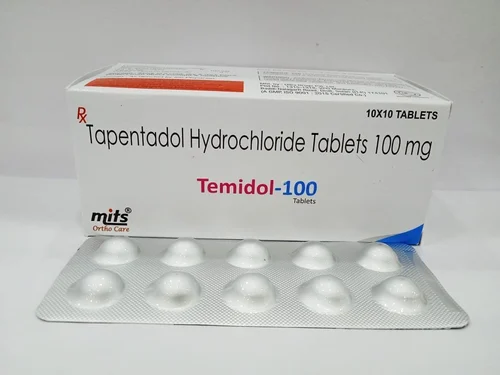
Tapentadol is used for the treatment of moderate to severe pain for both acute (following injury, surgery, etc.) and chronic musculoskeletal pain.
Availability: In Stock
Tapentadol 100mg, brand named Nucynta among others, is a centrally acting opioid analgesic of the benzenoid class with a dual mode of action as an agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Analgesia occurs within 32 minutes of oral administration and lasts for 4–6 hours.
It is similar to tramadol in its dual mechanism of action; namely, its ability to activate the opioid receptor and inhibit the reuptake of norepinephrine. Unlike tramadol 100mg, it has only weak effects on the reuptake of serotonin and is a significantly more potent opioid with no known active metabolites. Tapentadol is not a pro-drug and therefore does not rely on metabolism to produce its therapeutic effects; this makes it a useful moderate-potency analgesic option for patients who do not respond adequately to more commonly used opioids due to genetic disposition (poor metabolizers of CYP3A4 and CYP2D6), as well as providing a more consistent dosage-response range among the patient population.
The potency of tapentadol 100mg is somewhere between that of tramadol and morphine, with an analgesic efficacy comparable to that of oxycodone despite a lower incidence of side effects. It is generally regarded as a moderately strong opioid. The CDC Opioid Guidelines Calculator estimates a conversation rate of 50mg of tapentadol equaling 20mg of oral morphine sulfate in terms of opioid receptor activation.
Tapentadol 100mg was approved by the US FDA in November 2008, by the TGA of Australia in December 2010, and by the MHRA of the UK in February 2011. In India, the Central Drug Standard Control Organisation (CDSCO) approved tapentadol immediate-release (IR) preparations (50, 75, and 100 mg) for moderate to severe acute pain and extended-release (ER) preparations (50,100,150, and 200 mg) for severe acute pain in April 2011 and December 2013 respectively.